Vapor Pressure of Binary Solutions

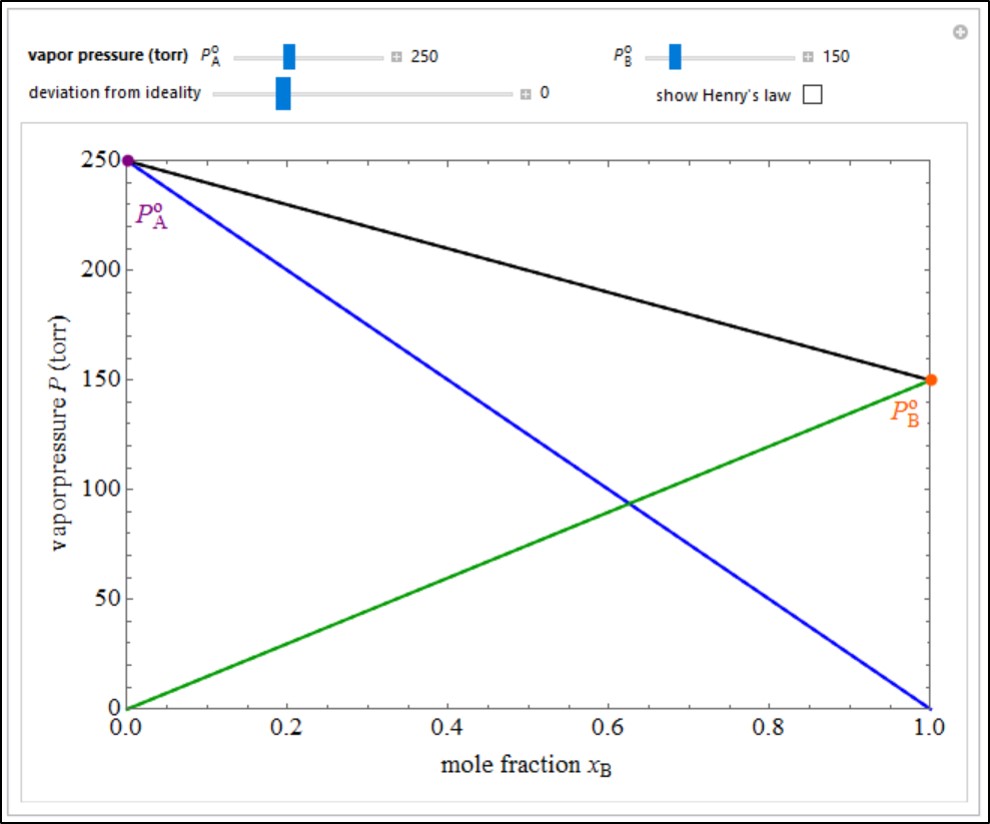
An ideal solution of two liquids A and B obeys Raoult’s law, which states that the partial pressure of each component is proportional to its mole fraction: PA = PAoxA and PB = PBoxB, where PAo and PBo are the vapor pressures of the pure components at a given temperature. The total pressure is then P= PA + PB = PAo(1 – xB) + PBo. Most real solutions exhibit deviations from Raoult’s law. A positive deviation is characterized by PA > PAoxA and PB > PBoxB and indicates that the attractive interactions between like molecules is greater than that between A and B molecules. A negative deviation has PA < PAoxA and PB < PBoxB, implying stronger mutual interactions between unlike molecules. The curves shown in the graphic are qualitative approximations to the actual dependence of vapor pressures on composition. The blue and green curves represent the partial pressures of A and B, respectively, while the black curve shows the total vapor pressure. The dashed lines refer to the hypothetical ideal behavior of the corresponding vapor pressures. For nonideal solutions, Raoult’s law is asymptotically approached for xA or xB ≈ 1. Dilute solutions, on the other hand, are approximated by Henry’s law: the linear relations PA ≈ KAxA for xA ≈ 0 and PB ≈ KBxB for xB ≈ 0. Check “show Henry’s law” to show this behavior, dotted purple and orange lines represent Henry’s law. Set the vapor pressures of the liquids and the deviation from ideality with sliders. Move the mouse over a curve to see its label (real or ideal).
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
Author: S. M. Blinder, modified by Rachel Baumann and John L. Falconer. Open content licensed under CC BY-NC-SA
View the source code for this simulation
