Immiscible Liquids on Pressure-Composition Diagram

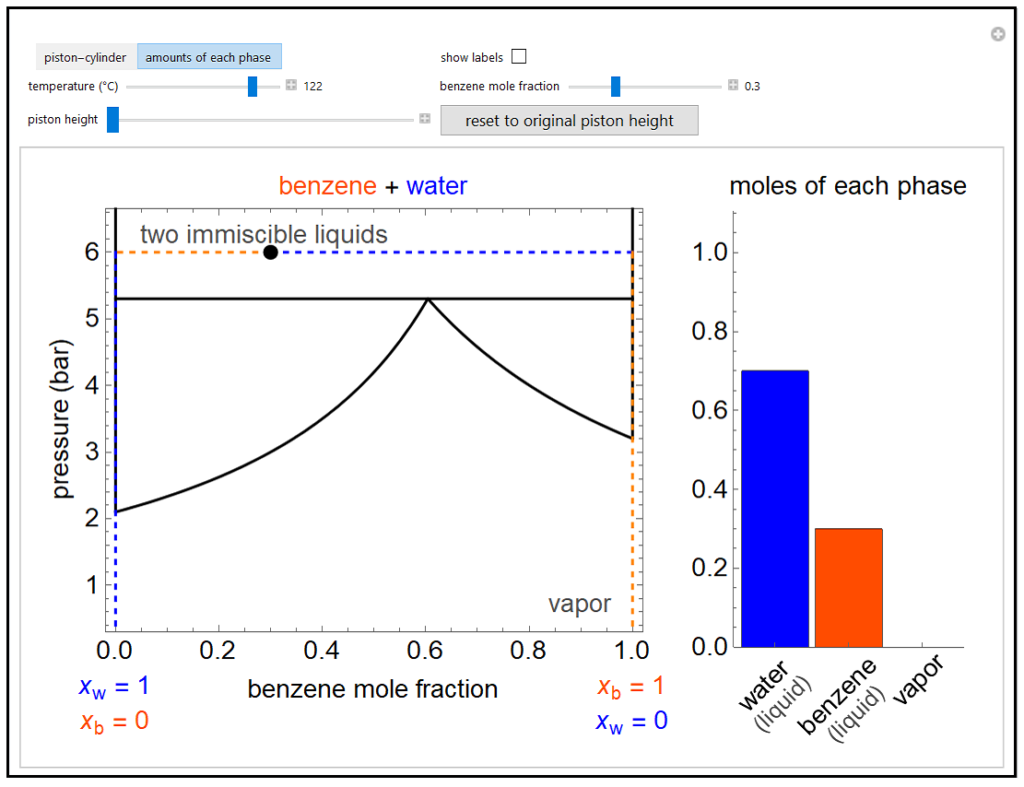
The pressure-composition phase diagram for two immiscible liquids, benzene and water, is at constant temperature. When the temperature is changed with a slider, the saturation pressures change. The overall benzene mole fraction can also be changed with a slider. The bar graph shows the moles of liquid water (blue), liquid benzene (orange), and vapor (green), which contains both components. The system contains one mole total. You can change the piston height to change the pressure and the amounts in each phase. At a given temperature, all three phases co-exist at only one pressure. When the piston height increases at this pressure, one of the liquid phases completely evaporates before the pressure decreases. Selecting “piston-cylinder” instead of “amounts of each phase” shows a representation of a piston-cylinder with the volume of each phase on a log scale.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation
