Compressed-Gas Spray

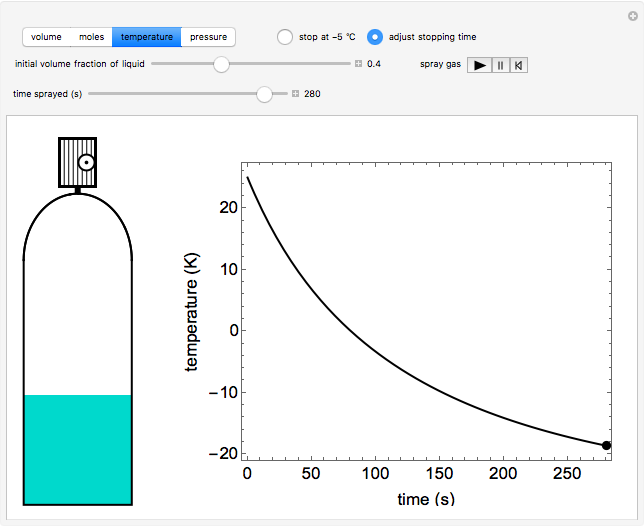
Compressed-gas dusters spray a gas such as difluoroethane (DFE) to remove dust from electronics. When gas exits the valve, liquid DFE in the container vaporizes to maintain vapor-liquid equilibrium. The energy to vaporize the liquid is obtained by cooling the remaining liquid; the container is modeled as adiabatic in this simulation. Decreasing the liquid temperature decreases its saturation pressure, which lowers the driving force, and thus the gas flow rate decreases. For smaller “initial volume fractions of liquid” (change with the slider), the liquid cools faster. Select a plot (volume, moles, temperature, or pressure) using the buttons to display how that property changes with time. Animate the duster by clicking the play button next to “spray gas.” Spray continues until the temperature reaches -5°C (if stop at -5°C is selected); otherwise the spray stops at the time selected by the “time sprayed” slider when “adjust stopping time” is selected. In either case, the black dot(s) show the conditions of the duster on the plot. The liquid and vapor DFE are assumed to be in equilibrium at all times. As the spray time increases, the adiabatic approximation becomes less accurate.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author(s): Rachael L. Baumann
View the source code for this simulation
