Multiple States in an Isothermal CSTR

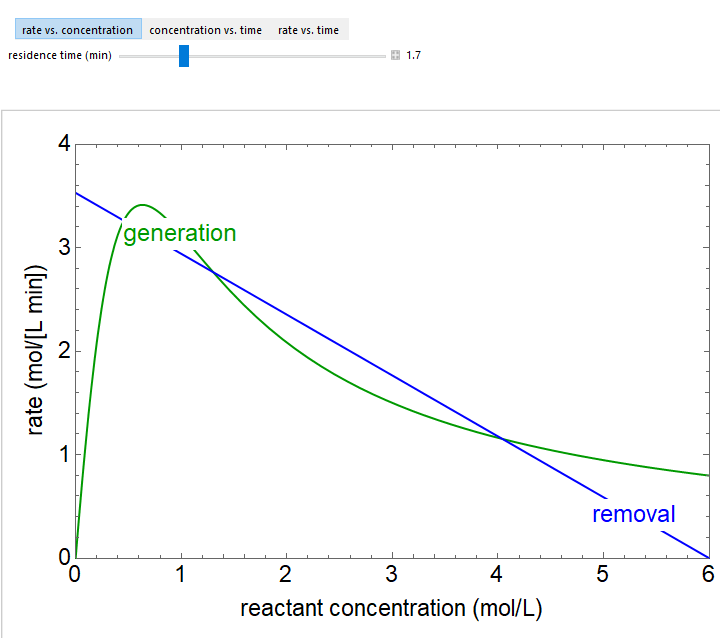
The reaction \( A \rightarrow B \), for which the kinetics are nonlinear, is carried out in an isothermal, continuous stirred-tank reactor (CSTR). Over a range of residence times, as shown in the rate versus reactant concentration plot, the reactor has three steady-state solutions, of which two are stable. The rate versus concentration plot shows the nonlinear rate dependence on concentration (green curve) and the mass balance (blue line). You can vary the reactor residence time in the rate versus concentration plot to determine conditions where either one or three steady states exist. The concentration versus time and the rate versus time plots show the approach to steady state for a user-selected initial reactant concentration in the reactor. These plots are obtained by solving the unsteady-state mass balance, and show that the concentration approaches one of the two stable states (when three steady-state solutions exist), depending on whether the starting concentration is above or below the concentration of the unstable state.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation
