CSTR That Loses Cooling

Description
Instructional video
Description
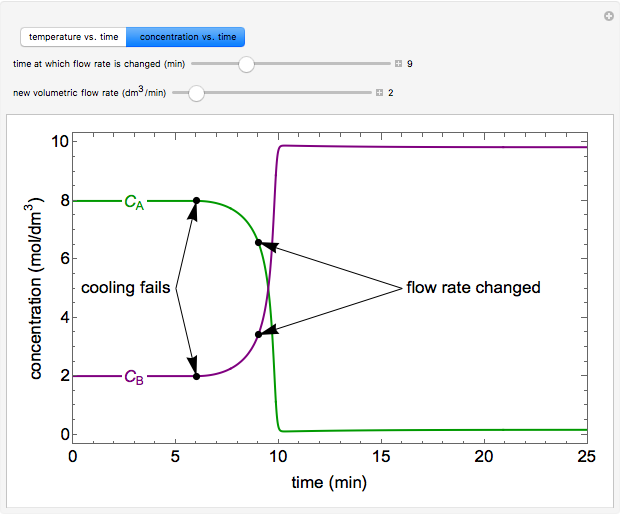
The exothermic reaction A –> B takes place in a continuous stirred-tank reactor (CSTR) with a cooling jacket. The reactor runs at steady state when the cooling fails at six minutes. After a few minutes, the chemical engineer notices that the reactor temperature is increasing because of the cooling failure. In order to prevent the reactor temperature from getting too high, should the engineer increase or decrease the feed flow rate?
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author(s): Rachael L. Baumann
View the source code for this simulation
Instructional video
