Batch Reactor with Multiple Reactions

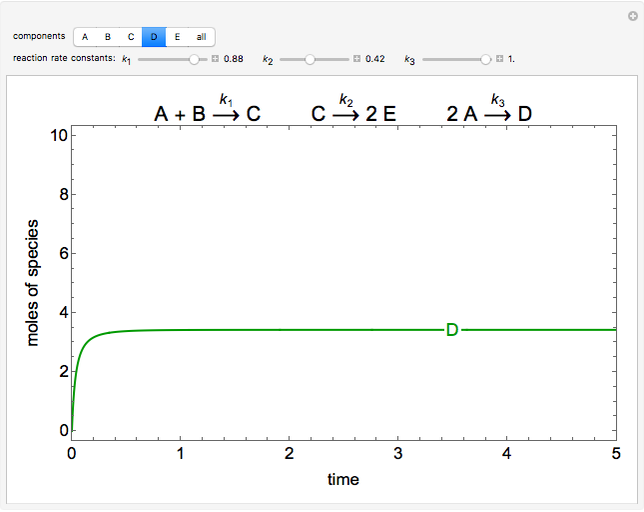
The concentrations of five species are plotted as a function of dimensionless time, for three irreversible, elementary, liquid-phase reactions in an isothermal batch reactor. Use sliders to change the dimensionless rate constants for each reaction. The initial amounts of A and B in the reactor are 10 mol and 5 mol, respectively. Use buttons to plot just one concentration or all five together. The reactions are: \[ A \rightarrow B + C \] \[ C \rightarrow 2E \] \[ 2A \rightarrow D \]
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation
