Entropy: Interactive Simulations
These simulations were prepared using Mathematica. Download the free Wolfram player, and then download the simulation CDF file (link given below or click on figure to download). Try to predict the behavior when a parameter changes before using a slider to change that parameter. Screencasts below explain how to use these simulations.
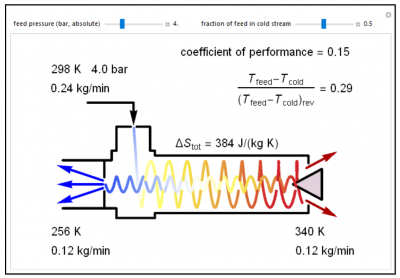
A vortex tube is a mechanical device used to separate a compressed gas into hot and cold streams. High-pressure air is fed into a Ranque-Hilsch vortex tube and air exits at 1-bar pressure; cold air exits the left side and hot air exits the right side. Use the “fraction of feed in cold stream” slider to adjust the fraction of feed in the cold stream (left side); this adjusts the throttle valve on the right side (triangle). Use the “feed pressure (bar, absolute)” slider to modify the inlet pressure. The color of the flowing gas indicates its temperature; blue is cold and red is hot. The coefficient of performance and isentropic efficiency are shown in the top-right corner. The coefficient of performance equals the isentropic efficiency multiplied by the fraction of feed gas in the cold stream.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- A Ranque-Hilsch vortex tube splits a high-pressure gas into a cold stream and a hot stream at lower pressure. Suppose the low-temperature stream is at 269 K for a feed pressure of 2.4 bar. If the feed pressure increases how does the temperature of the cold stream change?
- A Ranque-Hilsch vortex tube splits a high-pressure gas into a cold stream and a hot stream at lower pressure. Suppose the low-temperature stream is at 243 K and 1/3rd of the feed gas leaves in the cold stream. If the fraction of the gas leaving in the cold stream increases how does the temperature of the cold stream change?
- A Ranque-Hilsch vortex tube splits a high-pressure gas into a cold stream and a hot stream at lower pressure. How does the temperature change between the feed stream and the low-temperature stream compare to the temperature change if the gas was expanded adiabatically and reversibly?
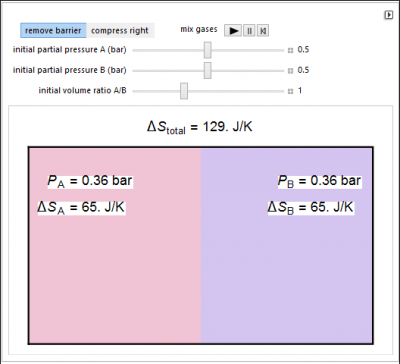
In this simulation, ideal gases A and B are mixed isothermally by keeping the total volume constant or by adding gas A to gas B so the final volume is the same as the initial volume of B. Select the “mix gases” play button to initiate mixing. The total entropy change is the sum of the entropy changes of each gas. Gas A is colored red and gas B is colored blue, and when the gases mix, different shades of purple result, depending on the ratio of moles of each species. As the number of moles increases, the color becomes more intense.
Try to answer these questions before determining the answer with the simulation. We suggest that you write down the reasons for your answers.
- How does the total entropy change when the initial partial pressure of A increases?
- If 1 mol of A and 1 mol of B, each at 0.5 bar, are mixed at constant temperature so that the final pressure is 1 bar, does entropy increase?