Fugacity Dependence on Pressure in an Ideal Binary Mixture

Description
Instructional video
Description
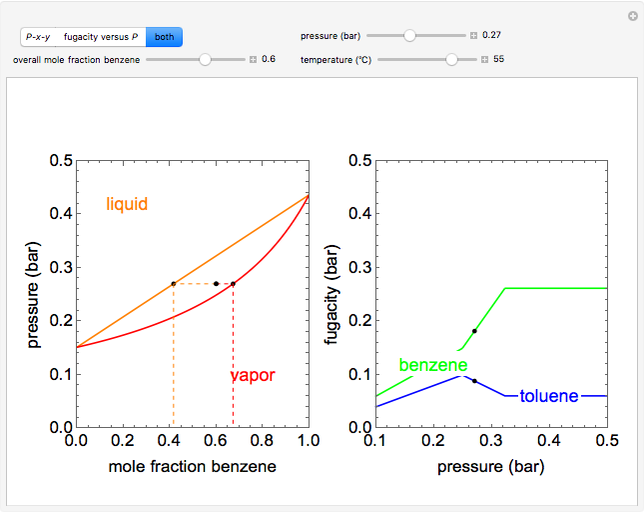
This simulation shows how the fugacities of benzene (B) and toluene (T) change with pressure and composition at constant temperature. The liquid mixture is modeled as an ideal solution and the gas phase is ideal, so Raoult’s law models vapor-liquid equilibrium. Use sliders to vary the temperature and overall mole fraction of benzene. Use buttons to view the pressure-composition diagram (P-x-y), the fugacity-pressure plot or both plots at once. Black dots on the diagrams represent the pressure (on the P-x-y diagram) or fugacity (on the fugacity plot) for the given temperature and pressure.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
View the source code for this simulation
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author(s): Neil HendrenView the source code for this simulation
Instructional video
