Heating Water in a Closed Vessel

Description
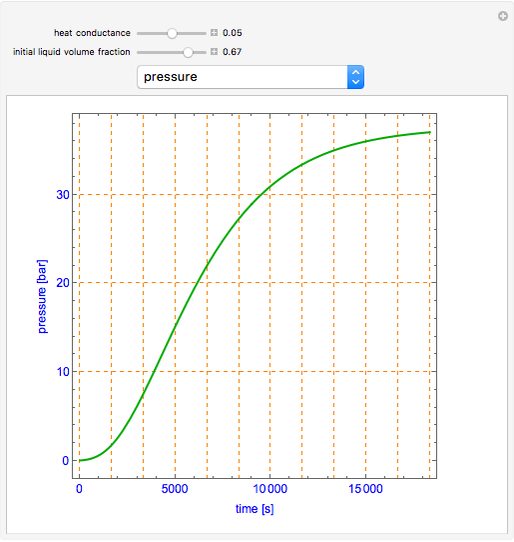
This simulation shows what happens to water, initially in liquid-vapor equilibrium at 300 K, if it is heated in a closed vessel with a given volume V. The heating medium is saturated steam at 520 K and the initial liquid volume fraction is Z0. Assuming that the system is always in a vapor-liquid equilibrium, the simulation plots the liquid mass, vapor mass, pressure, temperature, and heat input rate Q versus time. In addition, the dynamic behaviors of the liquid and vapor volumes and the liquid volume fraction Z = vl/V are displayed. Note that the liquid volume fraction increases upon heating for conditions of this simulation.
About
Authors: Housam Binous, Ismail Boukholda, Ahmed Bellagi. Open content licensed under CC BY-NC-SA.
View the source code for this simulation
