Temperature-Programmed Desorption

Description
Instructional video
Description
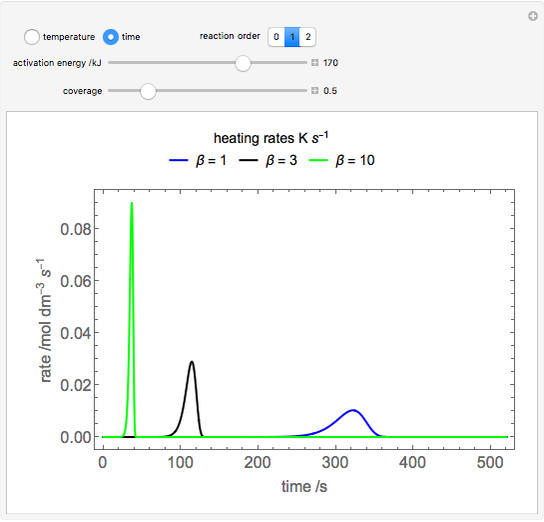
During temperature-programmed desorption (TPD), a molecule is adsorbed on a solid surface, the temperature is ramped linearly with time and the rate of desorption is measured. The rates of desorption are plotted versus either temperature or time (select with buttons) for three heating rates (1, 3 and 10 K/s). Select the desorption order with buttons; vary the desorption activation energy and the initial fractional surface coverage with sliders.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation
Instructional video
