Sensitivity of PFR to Model Parameters

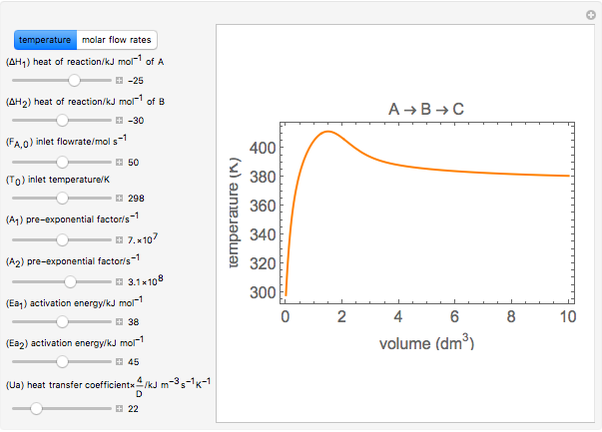
Gas-phase series reactions A –> B –> C take place in a non-isothermal plug flow reactor (PFR). Both reactions are first order. In this simulation you vary seven of the parameters used in the model and two of the feed conditions (temperature and flow rate) over relatively narrow ranges of values. Changing the feed temperature and flow rate shows how sensitive the reaction is to the feed conditions. You can vary each parameter and feed condition a maximum of ±10% as a way to illustrate the sensitivity of the model to the values of the parameters (parametric sensitivity) and what effects inaccuracies in their values have on the reactor conditions calculated. Since parameters used in a reactor simulation are not always known accurately, performing a parametric sensitivity analysis can be valuable.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author(s): Rachael L. Baumann
View the source code for this simulation
