Reaction in an Isothermal CSTR

Description
Instructional video
Description
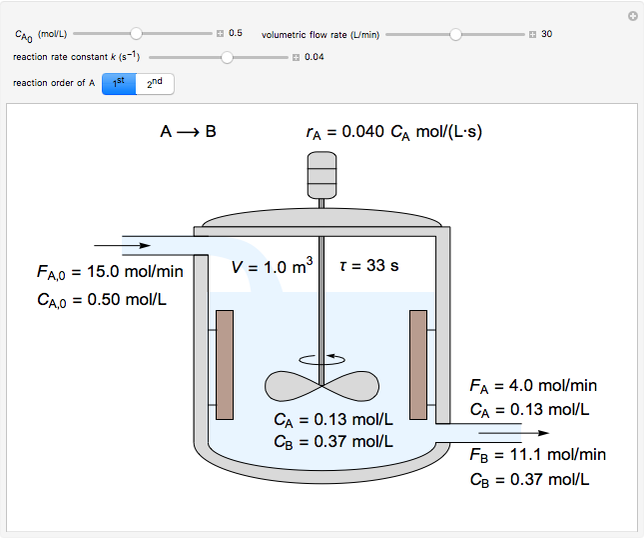
In this simulation, the liquid-phase reaction (A –> B) takes place in an isothermal, continuous stirred-tank reactor (CSTR). Use the sliders to set the feed concentration of A, (CA,0), the volumetric flow rate (v) and the rate constant (k). Select the reaction order with respect to A using the (1st) or (2nd) button. The rate constant has the same numerical value when the reaction order changes, but its units are different. The figure shows the feed molar flow rate (FA,0), the feed concentration (CA,0), the outlet molar flow rates (FA, FB) and the outlet concentrations (CA, CB). Note that the outlet concentrations are identical to the concentrations in the reactor. The reactor residence time (t = V/v) is also calculated.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
View the source code for this simulation
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author(s): Neil HendrenView the source code for this simulation
Instructional video
