Membrane Reactor for an Equilibrium-Limited Reaction

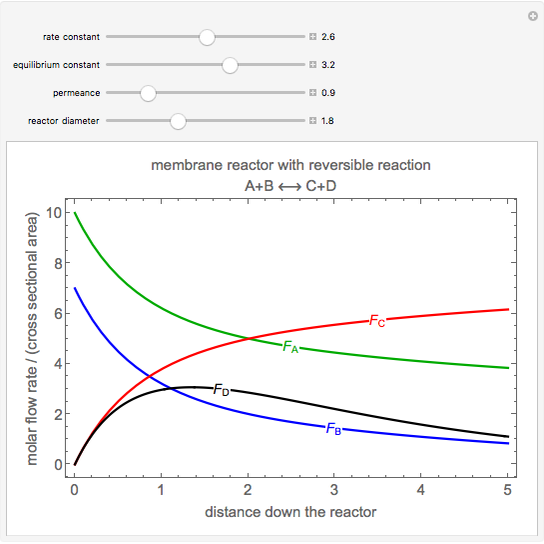
The elementary reversible reaction \( A + B \longleftrightarrow C + D \) is carried out in a tubular membrane reactor, which combines reaction with separation. Higher conversions are obtained for this equilibrium-limited reaction by selectively removing product \( D \) through the membrane, which is assumed to only permeate product \( D \). The permeate pressure of \( D \) (the pressure outside the tube) is assumed to be zero. The molar flow rates of reactants and products are divided by the cross-sectional area of the reactor tube in the plot to make the comparison easier when the reactor diameter changes. All values are dimensionless. Use sliders to change the forward reaction rate constant, equilibrium constant, and membrane permeance. When the membrane permeance is low, the reaction is equilibrium limited for large enough rate constants. Increasing the permeance shifts the equilibrium to the right to obtain higher conversion. Increasing the reactor diameter decreases the conversion because the permeation area per reactor volume decreases.
This simulation runs on desktop using the free Wolfram Player. Download the Wolfram Player here.
About:
This simulation was made at the University of Colorado Boulder, Department of Chemical and Biological Engineering. Author: Rachael L. Baumann
View the source code for this simulation
