Michaelis-Menten Enzyme Kinetics and the Steady-State Approximation

Description
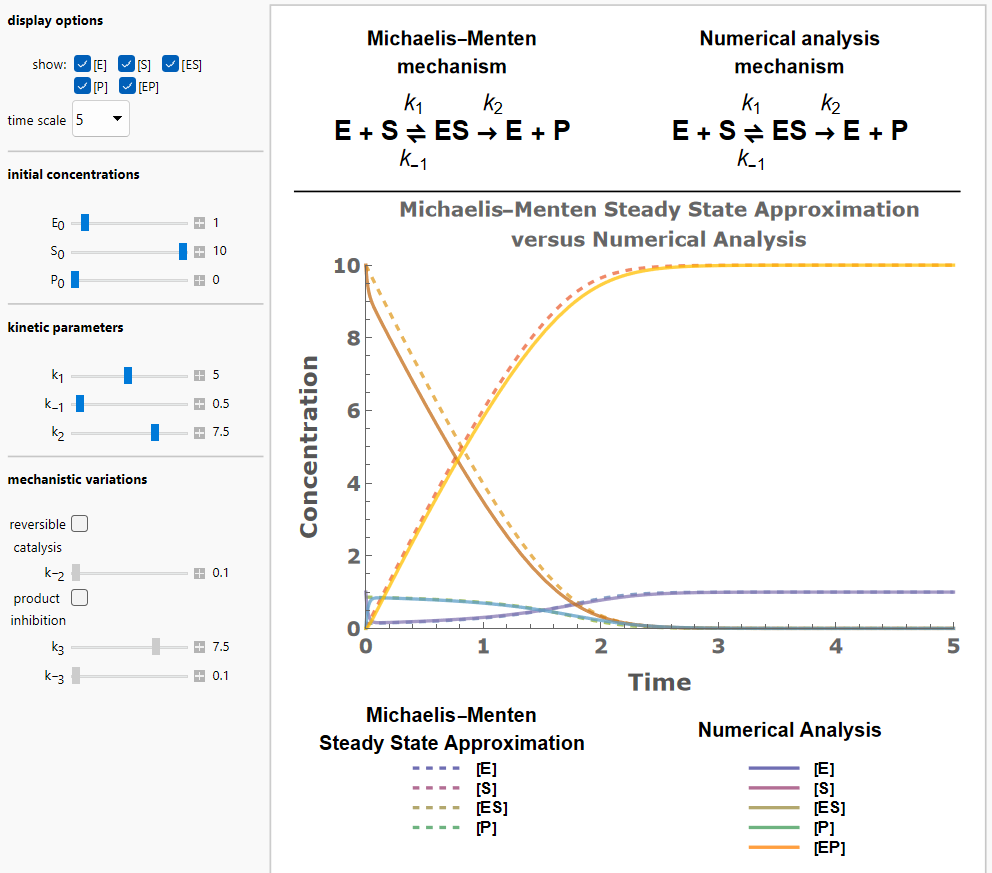
Michaelis–Menten enzyme kinetics is a model for rate equations that has a closed-form solution for the concentrations of reactants and products in an enzymatic reaction. Certain assumptions must be made to simplify the rate equations. In particular, the steady-state approximation assumes a negligible rate of change in the concentration of the enzyme-substrate complex during the course of the reaction. Furthermore, the Michaelis–Menten reaction mechanism presupposes that catalysis is irreversible and that the enzyme is not subject to any product inhibition. You can explore a wide variety of conditions, including initial concentrations, rate constants, and variations of the catalytic mechanism, and observe the effects of these combinations of parameters on the time evolution of the reactants and products. Both the results obtained via the Michaelis–Menten steady-state approximation (dotted lines), and those obtained via numerical integration (solid lines) are displayed. This allows you to get first-hand experience as to which combinations of parameters lead to deviations of the approximate solutions from the numerical solutions. This can give you an idea of conditions under which the Michaelis–Menten analysis is sufficiently accurate.
About
Author: Brian M. Frezza. Open content licensed under CC BY-NC-SA.
View the source code for this simulation
